Views: 0 Author: Site Editor Publish Time: 2025-10-30 Origin: Site









Aziridine crosslinkers are essential in material science, enhancing polymer properties. They improve adhesion, chemical resistance, and thermal stability, making polymers ideal for demanding applications in automotive, electronics, and coatings.
In this article, we’ll explore the mechanism of aziridine crosslinkers, focusing on how they bond polymer chains to form durable networks. Understanding this process is key to maximizing their industrial potential.
Aziridine crosslinkers are chemical compounds that contain the aziridine ring, a three-membered nitrogen-containing ring structure. This strained ring is highly reactive, making it an excellent candidate for crosslinking polymer chains. The aziridine ring can react with nucleophilic functional groups (such as carboxyl, amine, and hydroxyl groups), which leads to the formation of covalent bonds between polymer molecules, creating a network structure.
These crosslinkers are typically used in coatings, adhesives, and elastomers to enhance the material's performance. Their ability to form strong covalent bonds helps improve properties like heat resistance, chemical resistance, and mechanical strength.
Aziridine crosslinkers are widely used in:
● Coatings: To improve durability and resistance to corrosion and environmental stress.
● Adhesives: To enhance bond strength and thermal stability, crucial for automotive, aerospace, and construction industries.
● Elastomers: To increase flexibility and resistance to wear and tear.
By facilitating the formation of a crosslinked polymer network, aziridine crosslinkers ensure that materials perform better under challenging conditions, such as exposure to heat, chemicals, and mechanical stress.
The core feature of aziridine crosslinkers is their aziridine ring, a highly strained three-membered ring structure containing one nitrogen atom. This strain makes the ring unstable and highly reactive, enabling it to easily open and form bonds with nucleophilic groups, such as carboxyl or amine groups, present in the polymer. The chemical structure allows aziridine crosslinkers to efficiently connect polymer chains, forming a dense and durable network.
Aziridine crosslinkers contain functional groups like:
● Aziridine (C-N-C): The reactive site that facilitates ring-opening and crosslinking.
● Hydroxyl, Amine, and Carboxyl Groups: These groups on the polymer chain interact with the aziridine ring to form covalent bonds, which link the polymer chains together.
These functional groups play a critical role in the crosslinking process, influencing the rate and efficiency of crosslinking.
The mechanism of aziridine crosslinkers begins when the aziridine ring reacts with nucleophilic groups (such as carboxyl or amine groups) present in the polymer matrix. Upon contact, the aziridine ring opens, allowing the nitrogen atom to form a bond with the functional group. This reaction is often catalyzed by heat or a mildly acidic environment, which helps activate the aziridine crosslinker and accelerate the reaction.
The ring-opening of the aziridine involves the nitrogen atom attacking the active hydrogen of the nucleophilic functional group (e.g., -COOH or -NH2). This attack breaks the strained ring and leads to the formation of a new covalent bond between the polymer chain and the aziridine crosslinker.
This step is crucial because it allows the individual polymer chains to be chemically linked, creating a crosslinked structure that enhances the material's strength and stability.
As the reaction progresses, more aziridine molecules react with the polymer chains, creating a three-dimensional network. The result is a crosslinked polymer network, where the polymer chains are interconnected by covalent bonds formed by the aziridine crosslinkers. This network is responsible for the enhanced properties of the material, such as improved durability, heat resistance, and chemical stability.
Heat plays a significant role in accelerating the crosslinking reaction. While the reaction can occur at room temperature, applying heat speeds up the process and increases the crosslink density, leading to a stronger and more durable network. The temperature range for optimal crosslinking typically falls between 60°C and 120°C, depending on the specific formulation and desired properties.
Aziridine crosslinkers come in two main types:
● Polyfunctional: These crosslinkers have multiple aziridine groups and can form more complex networks. They are typically used for applications requiring high crosslink density and strength.
● Difunctional: These crosslinkers contain only two reactive aziridine groups, making them ideal for creating simpler, less dense crosslinked networks.
The choice between polyfunctional and difunctional aziridine crosslinkers depends on the specific requirements of the application, such as the desired mechanical properties, flexibility, and resistance.
Both temperature and pH have a significant impact on the efficiency of the crosslinking reaction. Higher temperatures tend to accelerate the reaction, while certain pH levels can activate or deactivate the crosslinker. Typically, a mildly acidic pH (around 6 to 7) is preferred for the crosslinking reaction, as it helps facilitate the opening of the aziridine ring.
The concentration and dosage of the aziridine crosslinker are crucial for achieving the desired level of crosslinking. Typically, aziridine crosslinkers are added at concentrations of 2-3% by weight. Too little crosslinker will result in a weak network, while too much can cause excessive crosslinking, leading to brittleness.
One of the key benefits of aziridine crosslinkers is the enhancement of mechanical properties. By creating a crosslinked network, aziridine crosslinkers improve the strength, durability, and chemical resistance of the material. This makes them particularly useful in applications requiring high-performance coatings, adhesives, and elastomers.
Unlike some other crosslinking agents, aziridine crosslinkers are non-toxic and environmentally friendly. They do not release harmful by-products such as formaldehyde during the curing process, making them safer for both workers and the environment.
The versatility of aziridine crosslinkers allows them to be used in various industries, from automotive to electronics and packaging. Their ability to enhance the performance of polymers makes them indispensable in creating long-lasting, high-quality materials.
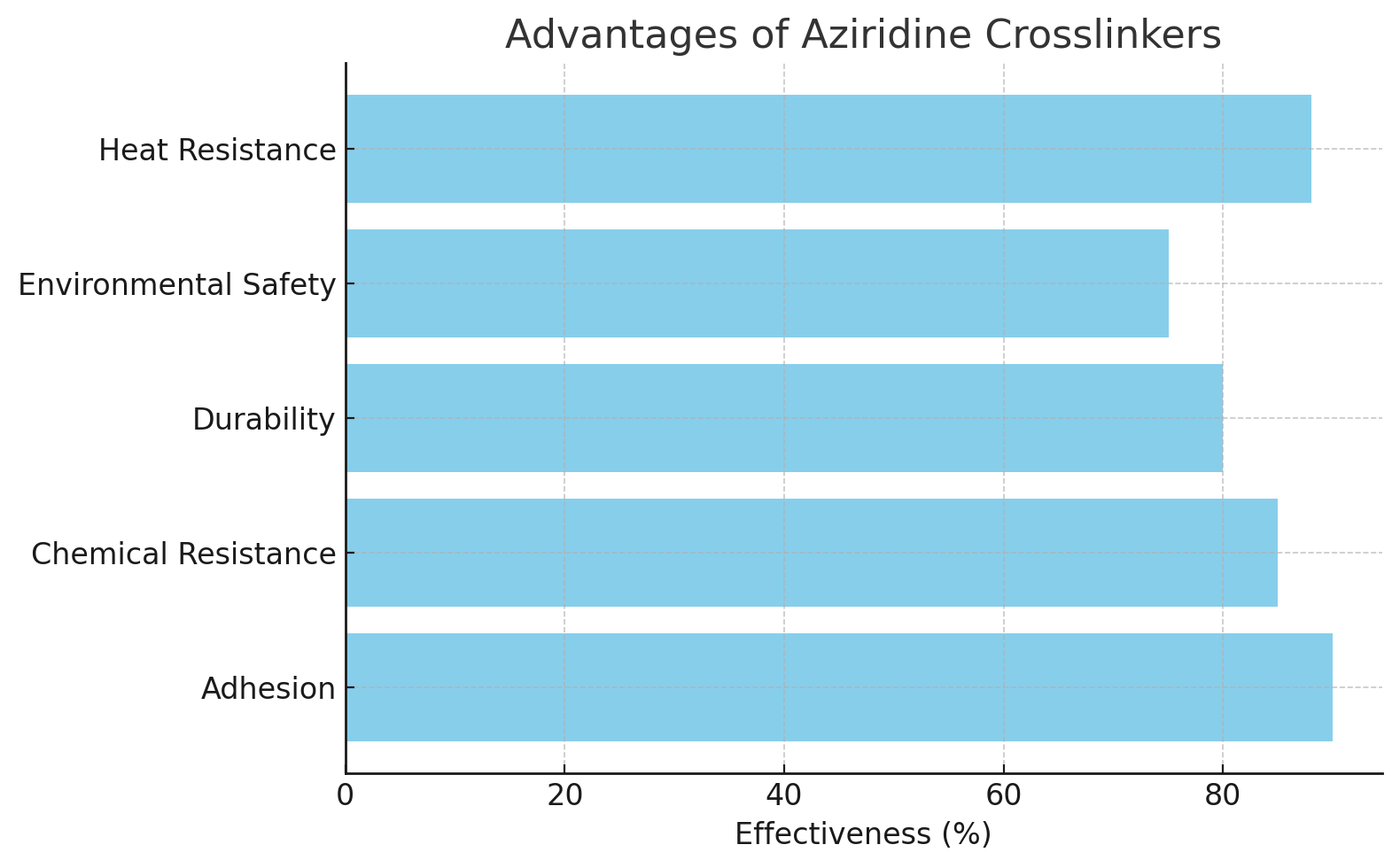
When compared to other crosslinkers such as melamines, isocyanates, and carbodiimides, aziridine crosslinkers offer distinct advantages. They tend to form more uniform networks and have better environmental profiles, as they do not release toxic by-products. Moreover, aziridine crosslinkers can be used in a wider range of formulations, making them more versatile than traditional alternatives.
Aziridine crosslinkers excel in coatings and adhesives, where they provide superior adhesion and chemical resistance. They also perform well in elastomers, where flexibility and resistance to wear are crucial.
While aziridine crosslinkers might be more expensive than some conventional agents, their efficiency in forming strong networks and their environmental benefits often outweigh the additional cost. In the long term, they provide better performance, leading to cost savings in material durability and maintenance.
When working with aziridine crosslinkers, it's essential to follow proper mixing procedures. The crosslinker should be added gradually and under continuous stirring to ensure uniform distribution within the polymer matrix.
The curing process plays a critical role in the final properties of the material. It’s essential to follow the recommended curing temperature and time to ensure optimal crosslinking. Typically, curing should occur at temperatures between 60°C and 120°C for several minutes.
Aziridine crosslinkers should be stored in a cool, dry place, away from direct sunlight. Most aziridine crosslinkers have a shelf life of 6-12 months when stored properly.
Aziridine crosslinkers are essential for enhancing polymer properties, making them indispensable for high-performance materials. They improve material strength, resistance, and longevity, benefiting industries like coatings, adhesives, and elastomers. By understanding their mechanism, businesses can maximize the potential of these crosslinkers in various applications. Companies like MSN Chemical offer products that provide environmentally friendly and versatile solutions, enabling businesses to optimize polymer formulations for superior performance.
A: An aziridine crosslinker is a chemical compound with a reactive aziridine ring, used to form covalent bonds between polymer chains, creating a durable and stable network.
A: Aziridine crosslinkers open their reactive ring upon contact with nucleophilic groups, such as carboxyl or amine groups, forming covalent bonds and crosslinking the polymer chains.
A: Aziridine crosslinkers are used in coatings because they enhance adhesion, chemical resistance, and durability, making coatings more resistant to wear and environmental stress.
A: Aziridine crosslinkers offer improved mechanical strength, resistance to heat and chemicals, and provide an environmentally friendly solution for crosslinking polymers.
A: Compared to other agents, aziridine crosslinkers form stronger, more durable networks and are safer, as they do not release harmful by-products like formaldehyde.
A: Temperature, pH, and crosslinker concentration significantly impact the crosslinking process, affecting the speed and density of the polymer network.
A: Yes, aziridine crosslinkers are non-toxic and environmentally friendly, offering a safer alternative to other crosslinking agents that release harmful substances during the curing process.
